Abstract
Background: Venous thromboembolism is an important complication of cancer. Elevated biomarkers of hypercoagulability, such as D-Dimer and Prothrombin Fragment 1.2 (F1.2), are associated with an increased risk of cancer-associated VTE. However, the evolution of these biomarkers during the course of disease is incompletely understood. Moreover, it is unclear whether potential longitudinal changes in these biomarkers over time harbor additional prognostic information on VTE risk in the oncologic setting.
Aim: To define the role of longitudinal haemostatic biomarker trajectories for personalized prediction of VTE risk in patients with cancer.
Methods: In this prospective cohort study, 164 patients with active malignancy were enrolled at cancer diagnosis or at progression after complete or partial remission (median age: 62 years [51-69; female sex: n=79 (48%); tumor sites: lung n=58 (35%), brain n=48 (29%), pancreas n=33 (20%), colon n=22 (13%), stomach n=3 (2%); metastatic disease: n=72 (44%)). D-Dimer (median: 0.8 µg/mL [25th-75th percentile: 0.4-2.0]), F1.2 (227 pmol/L [166-332]), and others (to be presented at the meeting) were measured at study inclusion, as well as during 637 follow-up visits each 1 month apart, for a planned number of 7 total visits (median number of visits: 5 [4-7]). Primary endpoint of the study was a composite of symptomatic, objectively-confirmed, and independently-adjudicated deep vein thrombosis and/or pulmonary embolism (VTE) during the observation period of 250 days. Joint models of longitudinal and time-to-event data were implemented to study how the haemostatic biomarker trajectories associate with the risk of cancer-associated VTE.
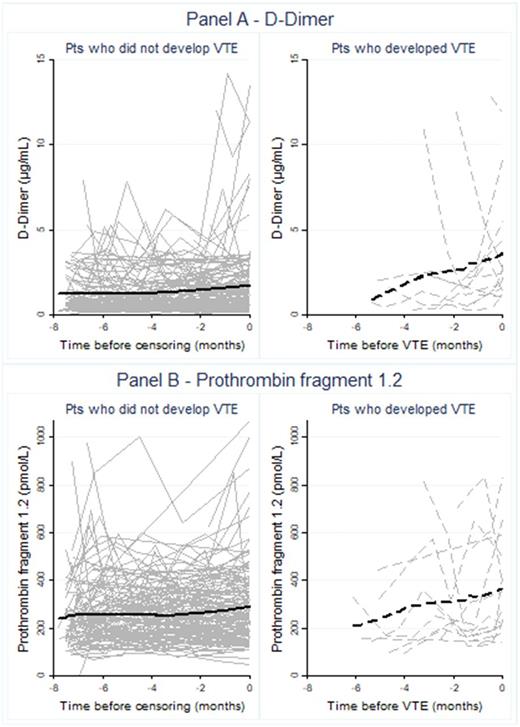
Results: The primary endpoint of VTE occurred in 18 patients, corresponding to a 250-day VTE risk of 12.1% (95% confidence interval (CI): 7.8-18.5). Graphical inspection of biomarker trajectories with a moving average suggested that levels of D-Dimer (Figure 1, Panel A) and F1.2 (Figure 1, Panel B) increased before the occurrence of VTE. Indeed, in linear mixed modeling, D-Dimer increased by 0.19 µg/mL/month (0.05-0.32, p=0.007) in patients who eventually developed VTE, but remained constant in patients who did not develop VTE (change per month=0.01 µg/mL, -0.09-0.9, p=0.97). Similarly, F1.2 increased by 9.8 pmol/L/month (0.2-19.5, p=0.045) in patients who developed VTE, while the corresponding increase in patients who did not develop VTE was smaller and not statistically significant (3.3 pmol/L/month, -1.2-7.9, p=0.155). In joint modeling, a doubling of the D-Dimer trajectory was associated with a 2.8-fold increase in the risk of VTE (Hazard ratio (HR)=2.79, 95%CI: 1.66-4.69, p<0.0001). A corresponding increase in F1.2 was associated with a 2.6-fold increase in VTE risk (HR=2.61, 1.02-6.74, p=0.046), respectively. While patients with metastatic cancer had higher levels of both D-Dimer (1.2 vs. 0.6 µg/mL, p<0.0001) and F1.2 (249 vs. 219 pmol/L, p=0.0003) than non-metastatic patients, the prognostic associations between the biomarker trajectories and VTE risk was highly comparable between these two patient groups (p for interaction=0.48, HR for D-Dimer trajectory in non-metastatic patients=3.16 (1.65-6.06, p=0.001), HR for D-Dimer trajectory in metastatic patients=2.51 (1.38-4.56, p=0.003); results for F1.2 not shown).
Conclusions: D-Dimer and F1.2 increase before the onset of cancer-associated VTE, but remain relatively constant over time in patients with cancer who do not develop VTE. So-called joint models can successfully link these biomarker trajectories to the prospective risk of VTE. These findings apply similarly to patients with metastatic and non-metastatic cancers. We conclude that the assessment of haemostatic biomarker trajectories in patients with cancer is a novel concept that could substantially advance the personalized assessment of VTE risk in the oncologic setting.
Pabinger: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Boehringer Ingelheim: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal